Am I the only one who wonders what the earth is REALLY made of? I remember being more concerned about my kids’ dummies falling on the floor in a public area than when they gummed a fistful of sand – why? Because it’s natural, I suppose. But what is it made of? For farmers (more than mothers) this question needs answers.

As always, we will relate what we find back to how it can help us grow more, bigger, better nuts.
How soils form
When the earth was forming, several different rocks were created. These rocks, made up of many different minerals, are breaking down over time, through chemical and physical weathering. They have now become part of the soil. The types of soil minerals determine the soil chemical properties, and are partly responsible for how fertile the soil will be.
Most of these minerals are in the clay part of soil. The rest of the soil is made up of air, water, organic matter, sand and silt.

Plants are the main source of food for humans, animals and birds and they get their nutrients from the soil. Therefore, most living things on earth depend on soil for their existence.
Although we have a good understanding of what makes up soil, there is still so much that we DON’T know, especially when it comes to the LIFE of soil – the micro-organisms that orchestrate and deliver well-rounded and wholesome fertility. Humans can put together water, chemicals, air and organic matter but we can’t make it fertile without that secret ingredient of LIFE.
Although supremely powerful, this life is also extremely fragile and when we add to or take away from the soil, we risk unbalancing a delicate structure, the consequences of which can be catastrophic.

Factors affecting soil formation
Soil forms continuously, but slowly, through 5 main interacting activities:
- Parent material (rocks) — these elements are the basis of soil. Granite type material often results in sandy, less fertile soil while basalt, under moist conditions, breaks down to form more fertile, clay soils.
- Living organisms (plants, micro-organisms like bacteria and fungi, insects, animals and humans) influence soil formation especially with regards to peat, humus or charcoal content.
- Climate
- Temperature affects the rate of weathering and organic decomposition. With a colder and drier climate, these processes can be slow but, with heat and moisture, they speed up.
- Rainfall dissolves some of the soil materials and holds others in suspension. The water carries or leaches these materials down through the soil. Over time this process can change the soil, affecting fertility.
- Topography
- The shape, length and grade of a slope affects drainage. The aspect of a slope determines the type of vegetation and indicates the amount of rainfall received. These factors change the way soils form.
- Soil materials are progressively moved within the natural landscape by the action of water, gravity and wind (for example, heavy rains erode soils from the hills to lower areas, forming deep soils). The soils left on steep hills are usually shallower. Transported soils include:
-
-
- alluvial (water transported)
- colluvial (gravity transported)
- aeolian (wind transported) soils.
-
- Soil properties may vary depending on how long the soil has been weathered. Minerals from rocks are further weathered to form materials such as clays and oxides of iron and aluminium.
How these factors interact produces an infinite variety of soils across the earth’s surface.
Soil Layers
As soils develop over time, layers (or horizons) form a soil profile. Most soil profiles cover the earth as 2 main layers—topsoil and subsoil.
Soil horizons are the layers in the soil as you move down the soil profile. A soil profile may have soil horizons that are easy or difficult to distinguish.
Most soils exhibit 3 main horizons:
- Horizon A —humus-rich topsoil where nutrient, organic matter and biological activity are highest (i.e. most plant roots, earthworms, insects and micro-organisms are active). Horizon A is usually darker than other horizons because of the organic materials.
- Horizon B — clay-rich subsoil. This horizon is often less fertile than the topsoil but holds more moisture. It generally has a lighter colour and less biological activity and may be heavier than Horizon A.
- Horizon C — underlying weathered rock (from which the horizons A and B form).
Some soils also have an O horizon mainly consisting of plant litter which has accumulated on the soil surface.
The properties of horizons are used to distinguish between soils and determine land-use potential.
Soil Content – in terms of minerals
Out of 92 naturally occurring chemical elements only 17 have been identified as essential elements (or nutrients), without which plants cannot grow and complete their life cycles. Elements essential for plant growth include:

As each one serves a purpose, is needed in various quantities, in diverse forms, at different times, and Agriculture has disrupted the natural balance of these elements, figuring out exactly WHAT we’ve removed and HOW to replace it (cost-effectively) is something we’re still trying to refine. For now, we can start with a basic understanding of each element – well, the ones we can supplement – and what it does for our mac nuts. But first, more understanding of soil fertility is necessary.
Soil fertility
Soil fertility is the ability of soil to sustain agricultural plant growth, i.e. to provide a healthy plant habitat that will result in sustained and consistent yields of high quality. There are a couple of concepts that I have had to master (well, you know, try and understand) before I felt qualified to share the next level of information (on each individual chemical / mineral).
A: Adsorption, desorption and ion exchanges. These things affect the movement of minerals through the soil to the plant’s roots. Without getting overly scientific, different elements have different charges (positive or negative) and this affects how they combine with, or stay separate from, other elements. These interactions affect movement and whether a plant can use the element in that state or not. These are not processes we can control but they are processes we affect.
Cation Exchange Capacity (CEC) is an indication of soil fertility. It is the total amount of positive charges that the soil can absorb. The larger the CEC value, the more cations the soil can absorb and make available to plants. Soil with a low CEC is less fertile as it cannot hold onto many nutrients. Typically, sandy soils have a CEC of 1 to 5, loams 4 to 10, clay loams 6 to 15 and clays 5 to 40. The CEC level in clay soils depends largely on soil pH.
Positively charged soil nutrients are: Potassium, Aluminium, Ammonium, Sodium, Magnesium, Calcium, Zinc, Manganese, Iron and Copper.
B: pH levels: I covered this nicely (even if I do say so myself ) in this article: https://www.tropicalbytes.co.za/2020-8-jaff-9/ (scroll to about 4/5th of the way through the story) but the crux of it is that extremes (both low and high pH) affect the movement and availability of nutrients. This index is one we can neutralise but we should do so with a wholistic and sustainable outlook as that all-important microbial system is very sensitive to pH levels.

To optimise the availability of all nutrients to mac trees, we need to maintain a soil pH of about 5 to 5,5.
C: Mineralisation and immobilisation.
Soil is full of life. It is often said that a handful of soil has more living organisms than people on planet Earth. Soil is the stomach of the earth – consuming, digesting, and cycling nutrients and organisms.
Living organisms present in soil include archaea, bacteria, actinomycetes, fungi, algae, protozoa, and a wide variety of larger soil fauna including earthworms, ants, insects and animals that spend all or part of their life underground. The links between soil organisms and how they impact the soil’s chemical and physical properties is complex. All of these are important in making up the environment we call soil and in bringing about numerous transformations that are vitally important to life.
Nutrient Cycling is the exchange of nutrients between the living and non-living parts of the ecosystem. The Carbon cycle is the measure of how plants and microbes absorb nutrients, and incorporate them into organic matter. There are two main processes in this cycle: Immobilization is when soil organisms take up mineral nutrients from the soil and transform them into microbial and plant tissues. The opposite process is mineralization, which is what happens when organisms die and release nutrients from their tissues. This process is very important in providing nutrients for plants to grow.

Understanding the individual nutrients and balances
Now that we have some of the fundamental science bedded down, we can look more closely at some of the nutrients essential to plant health, what they do and how we can help “stock the pantry”.
It is so important to understand that too much of a good thing can be bad (toxicity) – there is an optimum level of everything. Balance and sustainability of the overall environment is VITAL because, once that tower of jenga blocks comes tumbling down, we may as well walk away …
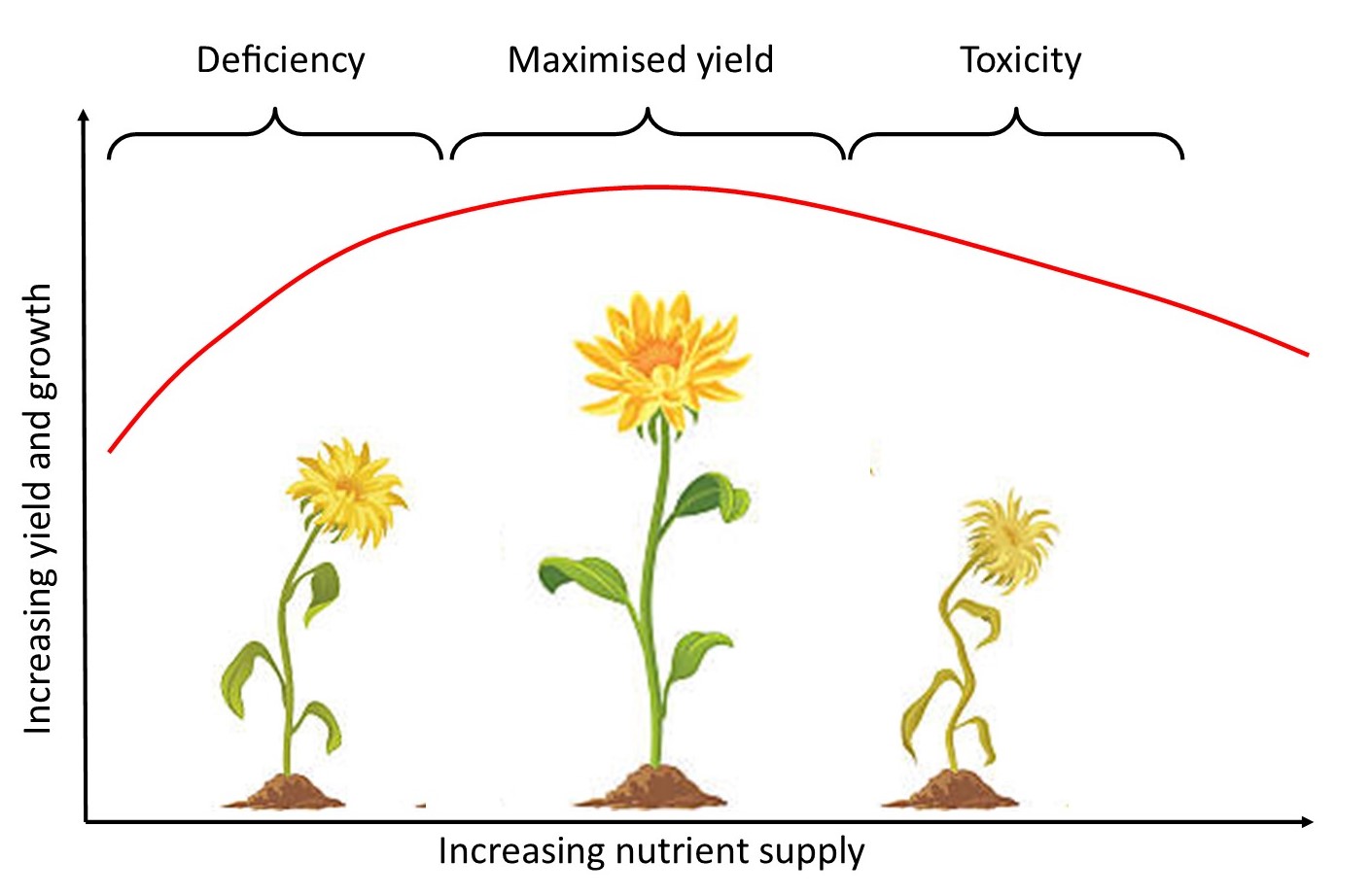
Too much can be just as bad as too little
We also need to understand that, in the soil, nutrients interact with one another which alters the availability to plants. The figure below (Mulder’s Chart) displays the various interactions that can occur.
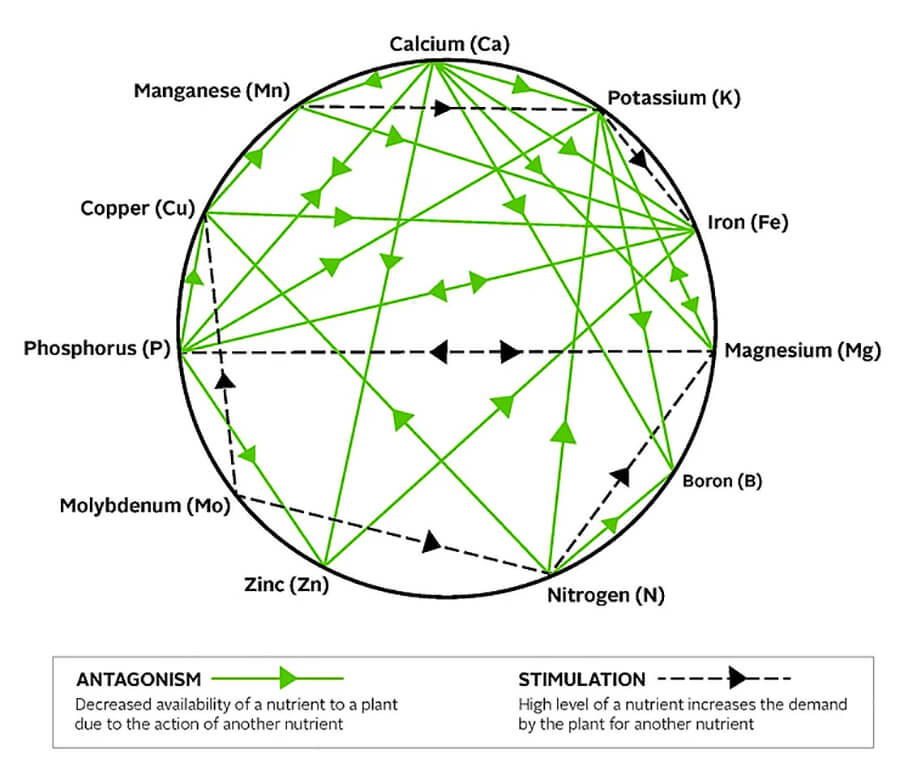
Antagonism: High levels of a particular nutrient in the soil can interfere with the availability and uptake of other nutrients. For example, high nitrogen levels can reduce the availability of boron, potash and copper; high phosphate levels can influence the uptake of iron, calcium, potash, copper and zinc; high potash levels can reduce the availability of magnesium. Thus, the application of high levels of nitrogen, phosphorus and potassium (NPK) can induce plant deficiencies of other essential elements.
Stimulation: This occurs when the high level of a particular nutrient increases the demand by the plant for another nutrient. For example, increased nitrogen levels create a demand for more magnesium.
That being said, let’s investigate each nutrient in isolation:

Nitrogen is an essential nutrient for plant growth, development and reproduction. Despite nitrogen being one of the most abundant elements on earth, nitrogen deficiency is probably the most common nutritional problem affecting plants worldwide because nitrogen from the atmosphere and earth’s crust is not directly available to plants.
Nitrogen is so vital because it is a major component of chlorophyll, the compound by which plants use sunlight energy to produce sugars from water and carbon dioxide (i.e. photosynthesis). It is also a major component of amino acids, the building blocks of proteins. Without proteins, plants wither and die. Some proteins act as structural units in plant cells while others act as enzymes, making many of the biochemical reactions, on which life is based, possible. Without nitrogen, there would be no life as we know it.
SOIL NITROGEN
Soil nitrogen exists in three general forms: organic nitrogen compounds, ammonium (NH₄⁺) ions and nitrate (NO₃⁻) ions.
At any given time, 95 to 99 percent of the potentially available nitrogen in the soil is in organic forms, either in plant and animal residues, in the relatively stable soil organic matter, or in living soil organisms, mainly microbes such as bacteria. This nitrogen is not directly available to plants, but some can be converted to available forms by micro-organisms. A very small amount of organic nitrogen may exist in soluble organic compounds, such as urea, that may be slightly available to plants.
The majority of plant-available nitrogen is in the inorganic forms; NH₄⁺ and NO₃⁻ (sometimes called mineral nitrogen).
NATURAL SOURCES OF SOIL NITROGEN
There are two sources of nitrogen that might eventually be used by plants: nitrogen-containing minerals in the soil and the vast storehouse of nitrogen in the atmosphere. The nitrogen in soil minerals is released during decomposition. This process is generally quite slow and contributes a small amount of plant-available nitrogen.
Atmospheric nitrogen is a major source of nitrogen in soils. In the atmosphere, it exists in the very inert N₂ form and must be converted before it becomes useful in the soil. The quantity of nitrogen added to the soil in this manner is directly related to thunderstorm activity, but most areas probably receive no more than 23kgs nitrogen/hectare per year from this source.
Bacteria such as Rhizobia that infect (nodulate) the roots of, and receive much food energy from, legume plants can fix much more nitrogen per year (some well over 110kgs per hectare). When the quantity of nitrogen fixed by Rhizobia exceeds that needed by the microbes themselves, it is released for use by the host legume plant. This is why well-nodulated legumes do not often respond to additions of nitrogen fertilizer. They are already receiving enough from the bacteria.
THE NITROGEN CYCLE
Nitrogen can go through many transformations in the soil. These transformations are often grouped into a system called the nitrogen cycle. Understanding the nitrogen cycle is vital to manage nutrient and fertilizers successfully. Because micro-organisms are responsible for most of these processes, they occur very slowly, if at all, when soil temperatures are below 10°C and/or are saturated.

Inorganic nitrogen is either in nitrate form or ammonium form. Plants are happy to use either. But, the ammonium form of nitrogen is more stable, and less prone to loss than the nitrate form.
Nitrogen is lost through:
- Leaching. Ammonium nitrogen turns into nitrate nitrogen fairly quickly, through a process called nitrification. The nitrate form of nitrogen is so soluble that it leaches easily when excess water percolates through the soil. This can be a major loss mechanism in coarse-textured soils where water percolates freely, but is less of a problem in finer-textured, more impermeable soils, where percolation is very slow.
- Denitrification. This is the conversion ofnitrate to atmospheric forms of nitrogen; Finer, impermeable soils tend to become saturated easily, and when micro-organisms run out of air (free oxygen supply) in the wet soil (they’re drowning), they get it by decomposing NO₃⁻. In this process, called denitrification, NO₃⁻ is converted to gas. The nitrogen is released in gaseous form and thereby lost to plants. When soils are warm and saturated for more than a few days, major amounts of nitrogen is lost through denitrification.
- Volatilization is the loss ofgaseous ammonia to the atmosphere. Some forms of fertiliser release the nitrogen as a gas (and therefore lost to the plant) under certain conditions such as warmth, high pH soil and a touch of moisture in the soil.
- Crop removal. This is more of an “opportunity cost” as the nitrogen in the harvested portions of the crop plant is removed from the field completely. This could have been recycled back into the system and turned into nitrogen.
PLANT NITROGEN NEEDS AND UPTAKE
Nitrate moves freely toward plant roots as they absorb water. Because plants require very large quantities of nitrogen, an extensive root system is essential to allowing unrestricted uptake. Plants with roots restricted by compaction may show signs of nitrogen deficiency even when adequate nitrogen is present in the soil.
Most plants take nitrogen from the soil continuously throughout their lives, and nitrogen demand usually increases as plant size increases. A plant supplied with adequate nitrogen grows rapidly and produces large amounts of succulent, green foliage. But, some plants may grow so rapidly when supplied with excessive nitrogen that they develop protoplasm faster than they can build sufficient supporting material in cell walls. Such plants are often rather weak and may be prone to mechanical injury. Something to consider with our often-brittle macadamia branches.
A yield of 3,5t of Nut-in-Shell /ha, would absorb about 63kg of nitrogen per hectare per year.

Phosphorous originates in rocks and is an essential nutrient for animals and plants. That’s because it is key in these functions:
- The replication of organisms (DNA, RNA).
- The plant needs phosphorous to build its root system, which is the basis for taking up other nutrients. Without enough phosphorus, plants are stunted and have low yields.
- It is the energetic currency of metabolism (ATP, NADP), controlling the storage and transfer of energy.
- It is the gatekeeper for moving compounds across cell walls (phospholipids).
Most phosphorus, used in agriculture worldwide, comes from mineral fertilisers. But the future of these mineral fertilisers has become uncertain. The first phosphorus price shock came in 2008, when the commodity price of rock phosphate, the raw material that is mined, spiked 800%. It is subject to uncertain supply because of the very uneven geological distribution of its deposits. Only five countries have combined control of 88% of remaining phosphate reserves. Morocco alone has 75% of estimated global reserves, some of it in the disputed territory of Western Sahara.
As if the importance, and dodgy supplies, of this element is not enough, phosphorous also has limited forms available to plants. This is because it binds easily to cations (positive molecules) in the soil which reduces its mobility and plant absorption. The soil pH also affects this binding. This is a very interesting video giving more detail on phosphorous availability in the soil: https://www.youtube.com/watch?v=P0QKPRK2_ws
We walk a tight-rope with this element as over-application can result in extreme environmental fallout – the most important of which is eutrophication – this is excessive build up of algae in surface waters.

Massive fish kills may occur due to anoxic conditions brought about by the decay of algae biomass stimulated by elevated inputs of phosphorous.
Thankfully, all this ‘bad news’ about phosphorus is not too concerning to the macadamia crop as it is an extremely efficient feeder, especially of this mineral, and therefore needs very little (if any) supplementation. Read more about this in the TropicalBytes article on Water Management: https://www.tropicalbytes.co.za/2020-4-water/ In fact, phosphorus-poor soils induce the formation of proteoid roots in macadamias, which greatly increases the surface area available for nutrient absorption. Mac trees can also produce a specialised root exudate that makes phosphorus even more available to the tree.
NB: Over supply (phosphorus toxicity) will inhibit the growth of these feeder (proteoid) roots. Obviously, a lack of feeder roots is a severe issue with countless knock-on effects so, to use a good old Zulu saying; “hamba kahle” (go carefully), with this element.
A yield of 3,5t of Nut-in-Shell (NIS)/ha, would absorb about 3,5kg of phosphorus per hectare per year.
In the November TropicalBytes story, you’ll hear from Oom Jaff who is a gold mine of wisdom. For young trees he uses phosphorous acid (H₃PO₃) as a root stimulant and an anti-fungus. He explains that it is a source of phosphates only when the tree wants it. PO₃, which is not an available phosphorous source for the trees, is converted, by microbiomes in the soil, to PO₄, which the trees can take up. Remember that healthy soil micro-life is an essential ingredient in this recipe. Oom says that, by adding H₃PO₃ to the irrigation water, he has helped many trees recover from phytophthora.

Carbon occurs naturally in the atmosphere and, unlike nitrogen and phosphorus, the plant uses it directly from that source, as carbon dioxide (CO₂), absorbed during photosynthesis. Without it, photosynthesis wouldn’t function and there would be no life as we know it.
Soil organic carbon also occurs naturally and, although plants don’t use it directly, it is essential as a part of the carbon cycle. Soil carbon fuels micro-organisms and these creatures perform more essential functions for our plants than we’ll ever know, or appreciate.
Carbon is the main element present in soil organic matter, on average making up 58% by weight.
Organic carbon influences many soil characteristics including nutrient and water holding capacity, nutrient cycling and stability, improved water infiltration and aeration.
Soil carbon is also important for soil bacteria. These soil-based microflora form microaggregates in the soil by binding soil particles together with their secretions. These microaggregates are like the building blocks for improving soil structure. Improved soil structure increases water filtration and increases water holding capacity of the soil. Many bacteria produce a layer of polysaccharides or glycoproteins that coat the surface of soil particles. These substances play an important role in cementing sand, silt and clay soil particles into stable microaggregates that improve soil structure.

Potassium also comes from rock materials. Soil reserves are generally large but mostly unavailable for plant uptake. As with most minerals it is found, in three forms, in the clay, sand and silt parts of soil:
- Primary minerals – in this form, plants cannot take it in.
- Compounds – as these compounds break down, potassium slowly becomes available to the plants. Recent investigations have shown that organic exudates (substances secreted by a plant, bacteria, fungi or other life forms) play a key role in releasing otherwise unavailable potassium from potassium-bearing minerals.
- Solutions – this is where plants get their usable potassium from. Suitable soil moisture means greater potassium availability as the element moves towards the roots in water. BUT air is also essential for root respiration and the uptake of potassium so saturated, over-wet soils are as unhealthy as dry ones. Loss of potassium due to leaching, in very wet soils, is also a factor to consider.
Potassium has some very important roles in plant health:
- Most importantly, it is essential for fruit GROWTH.
- Regulation of water balance – this is done by influencing water movement and controlling the opening and closing of stomata. This means that potassium is vital in addressing drought, salinity issues and resistance to pests and diseases.
- Synthesis and movement of sugars, starches and oils. NB: it is therefore VERY important for nut yield and quality.
To address potassium deficiencies or replace potassium resources in the orchards, we generally use a form of potash. In applying this supplement, balance is important: especially with calcium and magnesium. Too much of one inhibits the availability of the others.
If your soil pH is already high, then consider using something that is acid-based. More on this in our November TropicalBytes story, on “Oom Jaff” … he uses potassium nitrate and fulvic acid in a foliar spray before flowering. The potassium nitrate stimulates growth while the fulvic acid is the organic additive that makes the inorganic potassium available to the tree.

When I think of sulphur, I think of boarding school. For some reason we used to suffer from boils too often for comfort and the nurse’s cure was sulphur – pure, yellow, stinky powder – that we were allowed to mix with syrup so we could choke it down. I’m interested to see what our macs use it for …
Sulphur seems to be everywhere – it comes from rocks, specifically volcanic rock. It is a part of many minerals including iron pyrites, galena, gypsum and Epsom salts. All living things contain sulphur and, when fossilised, the sulphur remains present. Since industrialization, a lot of soil sulphur comes from sulphur dioxide in air pollution (from burning fossil fuels).
Organic sulphur is mineralised into an inorganic form and plants can take it up as sulphate. It is relatively mobile in the soil and other nutrients have little effect on its mobility towards the roots.
Although it is an important component of proteins and chlorophyll, macs don’t need very much (lucky buggers). There is normally enough available in the atmosphere and the soil, and many supplementary additives (like gypsum and superphosphate) have enough to make up any shortages. Sandy soils, where leaching of everything is a concern, may require some attention.

Soils derived from rocks like limestone or marl will tend to have higher calcium levels than those derived from shale or sandstone. Although calcium is not very mobile in the soil, or in plant tissue, and therefore a continuous supply is essential, the feeder roots of mac trees are very efficient in absorbing calcium.
Soil pH generally provides a good indication of calcium levels in soils. Alkaline soils generally contain more calcium than acid soils. Calcium is present in adequate amounts in most soils.
Calcium is essential for many plant functions. Some of them are:
- Proper cell division and elongation
- Proper cell wall development
- Nitrate uptake and metabolism
- Enzyme activity
- Starch metabolism
The choice of calcium product depends on the effect required. Lime is normally used when soil pH and calcium levels are both low. Dolomite is normally used when soil pH, calcium and magnesium levels are all low. Gypsum works well when pH is within the right range but the soil calcium level is low.
Oom Jaff (Nov story) shared some interesting insight as to why he sprays calcium and fulvic acid before fruit season; lessons were learnt in the apple and peach markets where fruit was going brown on the inside (called ‘biterpit’) because of a lack of calcium. Farmers used to spray calcium-arsenate although that has since been replaced with a special preparation that excludes arsenic. Plant cells, strengthened by calcium, are able to support the movement of water (and nutrients) better, resulting in well-fed fruit. Oom adds boron to his foliar feeds because it brings mobility and enhances the transfer of nutrients needed by the developing fruit.
A yield of about 3,5t of Nut-in-Shell /ha, would absorb about 35kg of calcium per hectare per year.

Macs need good iron levels. It plays a critical function in the production of chlorophyll and that is why deficiencies are so easily identified by the yellowing of the intervein areas of the leaf. Iron is generally abundant in the soil but high soil acidity and/ or high phosphorus levels will result in low iron accessibility (iron-chlorosis). pH levels that are too high also impact iron availability – above 7.0 pH, iron will bond with minerals like magnesium, calcium, zinc and manganese, making it insoluble and therefore unavailable to the tree.
It is essential that iron levels are maintained correctly because chlorophyll is energy and this is required to set a crop and to transform water into oil in the nuts.
Low soil temperatures also impact iron assimilation: at soil temperatures below 13°C, root activity starts to slow down: below 5°C, iron uptake will stop completely. This is why new growth occurring after a very cold winter will often show iron deficiency symptoms.

Magnesium is another nutrient involved in the production of chlorophyll – here it helps to trap light energy and convert it to chemical energy (sugars). It also regulates the uptake of other nutrients.
Magnesium is mobile in the soil and relatively easily absorbed into the plant. It also moves around the plant freely to address deficiencies in different parts.
Magnesium is one of those nutrients that are very sensitive to the presence or imbalance of other elements, especially calcium and potassium. An excess of one can reduce the availability of another.

Boron’s most important function lies in cell division and growth eg: flowers, nuts, shoots and root tips. This makes it VERY important to the productive mac orchard but getting the supply right is a tightrope as there is a fine line between deficiency and oversupply.
pH levels also affect boron with leaching being the main problem in acidic soils and a lack of availability being the problem in soils that are too alkaline. Moisture also needs to be managed as very wet and very dry conditions also affect the accessibility of this nutrient by the plant.
Although boron is very mobile in the soil, its mobility in the plant is far lower and it does not move around easily to address shortages in different parts of the plant. As a result, there needs to be a constant but measured supply of this nutrient throughout the season.
Even though boron itself is not highly mobile, it does enhance the mobility of other nutrients. Oom adds boron to his foliar feeds for this reason.

Although Copper is very important for cell formation, it is an element you want to be careful with as it can impact soil life negatively. Mac tree deficiencies present in wavy branches – this may be because copper is important in the production of lignin, a chemical that gives strength to the growth of lateral branches. Copper also facilitates the transfer of energy in various tree processes such as photosynthesis and nitrogen metabolism – pretty important jobs! Thankfully, copper is one of the least mobile nutrients in the soil so it doesn’t leach easily.
Except for the South Coast, most macadamia growing areas don’t struggle with copper shortages. Copper-based fungicides, routinely sprayed by a number of farmers for husk rot etc, generally address any deficiencies without the need to use copper fertilisers.
If phosphorus levels are very high, soils are very sandy or excess nitrogen is in the soil, you may struggle with copper availability to the plant.
If copper levels in the soil are too high, you may experience iron deficiencies.

Zinc deficiency is common in macadamia orchards, as it is in a lot of agricultural fields. Soil zinc is often low, and shortages can be induced by lime and phosphorus applications. In high pH conditions, zinc is made unavailable through adsorption to clay or calcium carbonate. Soils high in phosphorus decrease zinc solubility. In waterlogged soils, zinc deficiency occurs because of the formation of sparingly soluble zinc compounds in the oxidized rhizosphere (region around plant roots).
Sandy and highly-leached, acid soils generally have low plant-available zinc. Mineral soils with low soil organic matter also exhibit zinc deficiency. In contrast, soils originating from igneous rocks are higher in zinc.
Zinc is an important component of various enzymes that are responsible for driving many metabolic reactions in all crops. Growth and development would stop if specific enzymes were not present in plant tissue. Carbohydrate, protein, and chlorophyll formation is significantly reduced in zinc-deficient plants. Therefore, a constant and continuous supply of zinc is needed for optimum growth and maximum yield.
Zinc plays a major role in the fertility of the female parts of the macadamia flowers. Zinc is also needed for new growth and it plays a role in phosphorus metabolism and in the regulation of the use of water.
Farmers have said that, when newly flushed leaves are smaller than normal, the chances are that there’s a zinc shortage.

To be honest, it’s been challenging to find much of interest with regards to this nutrient. I actually found more information on manganese toxicity rather than deficiency which, in my mind, indicates that we don’t need to worry about adding any to the soil. One of the causes of manganese toxicity is too much magnesium. Manganese is only needed in very small amounts and (healthy) soil usually contains enough.

Mmm … another one that seems to have a warning label with regards to toxicity … a Hawaiian study on nickel revealed that liming reduced the adverse effects of too much nickel so, as we are generally avid limers, we should be safe from too much nickel!
In the past, nickel (Ni) was not considered an important element for plant growth, but research has discovered that it is an essential element for plant growth. The normal range for nickel in most plant tissue is between 0.05-5 ppm. Due to its low requirements (often in parts per billion), it is found in sufficient levels as a contaminant in the soil, water, fertilizer, etc. Nickel deficiency is unusual and is often misdiagnosed as it initially shows no symptoms in plants. This explains why most labs do not test for it and why it is not included in most fertilizers.
Nickel is a component of some plant enzymes, most notably urease, which metabolizes urea nitrogen into useable ammonia within the plant. Without nickel, toxic levels of urea can accumulate within the tissue forming necrotic legions on the leaf tips. In this case, nickel deficiency causes urea toxicity.
Nickel is also used as a catalyst in enzymes used to help legumes fix nitrogen. There is evidence that nickel helps with disease tolerance in plants, although it is still unclear how this happens.

Although essential for vital metabolic functions like photosynthesis and the opening and closing of stomata, chlorine deficiencies are rare because so little is required by the plant. It is far more likely that chlorine toxicity causes issues.

Molybdenum, the last of the required micronutrients, is needed in the smallest quantities by plants. Molybdenum is an essential component in two enzymes that convert nitrate into nitrite (a toxic form of nitrogen) and then into ammonia before it is used to synthesize amino acids within the plant. Plants also use molybdenum to convert inorganic phosphorus into organic forms in the plant.
Molybdenum becomes unavailable as the growing medium pH decreases.
Molybdenum typically comes from most water soluble and some controlled-release fertilisers. A fertiliser program can be supplemented with molybdenum by applying a complete micronutrient fertiliser (which helps avoid micronutrient imbalances) or through single element applications such as sodium molybdate or ammonium molybdate. Very little molybdenum needs to be applied to correct a deficiency.

Because Silica improves cell strength and many farmers are reporting good results after adding it to their programmes, I’ve included it here. Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO₂, most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Mac shells seem to be hardened by the presence of silica which is one way to cut down on pest damage to kernels.
Here’s a summary of the detail above. And below that is a graphic representation of the deficiencies described.
| Absorbed Form | Main Function/s | Deficiency | ||
| Macronutrients | ||||
| Nitrogen | N | NO3–, NH4+ | Protein and enzyme component | General yellowing of leaves, stunted growth, often older leaves affected first. |
| Phosphorus | P | HPO4–, HPO42- | Membranes, energy, DNA | Difficult to visualize until severe. Dwarfed or stunted plants. Older leaves turn dark green or reddish-purple. |
| Potassium | K | K+ | Osmotic balance | Older leaves may wilt or look burned. Yellowing between veins begins at the base of leaf and goes inward from the leaf edges. |
| Calcium | Ca | Ca2+ | Cell structure | Fruit/flower and new leaves are distorted or irregular. When severe, leaves will be necrotic near the base. Leaves can be cupped downward.
Occurs more often at low pH. |
| Magnesium | Mg | Mg2+ | Chlorophyll, enzyme activation | Older leaves will turn yellow and brown around the edge of the leaf leaving a green center. May appear puckered.
Occurs more often at low pH. |
| Sulphur | S | SO42- | Protein and enzyme component | Yellowing leaves starts with younger leaves. |
| Micronutrients | ||||
| Iron | Fe | Fe2+, Fe3+ | Enzyme function, required for chlorophyll production | Yellowing between veins that start with younger leaves. Occurs more often at high pH. |
| Manganese | Mn | Mn2+ | Enzyme component | Yellowing between veins that start with younger leaves. Pattern is not as distinct as with Fe deficiency, may appear in patches or freckled. Occurs more often at high pH. |
| Zinc | Zn | Zn2+ | Enzyme component | Yellowing between veins of younger leaves. Terminal leaves may be rosette. Occurs more often at high pH. |
| Boron | B | H2BO3– | Cell wall | Terminal buds die. Light general yellowing. B requirements are very plant specific. |
| Copper | Cu | Cu2+ | Enzyme function | Dark green stunted leaves. Curled leaves often bend downwards. Sometimes wilted with light overall yellowing of leaves. Occurs more often at high pH. |
| Molybdenum | Mo | MoO42- | Enzyme function | Yellowing of older leaves and light green rest of the plant. It usually appears as N deficiency due to role in nitrate assimilation and in legumes in N-fixing bacteria. Occurs more often at low pH. |
| Chlorine | Cl | Cl– | Osmotic balance, plant compounds | Almost never deficient. Abnormally shaped leaves; Yellowing and wilting of young leaves. |
| Nickel | Ni | Ni2+ | Enzyme component | Almost never deficient. |
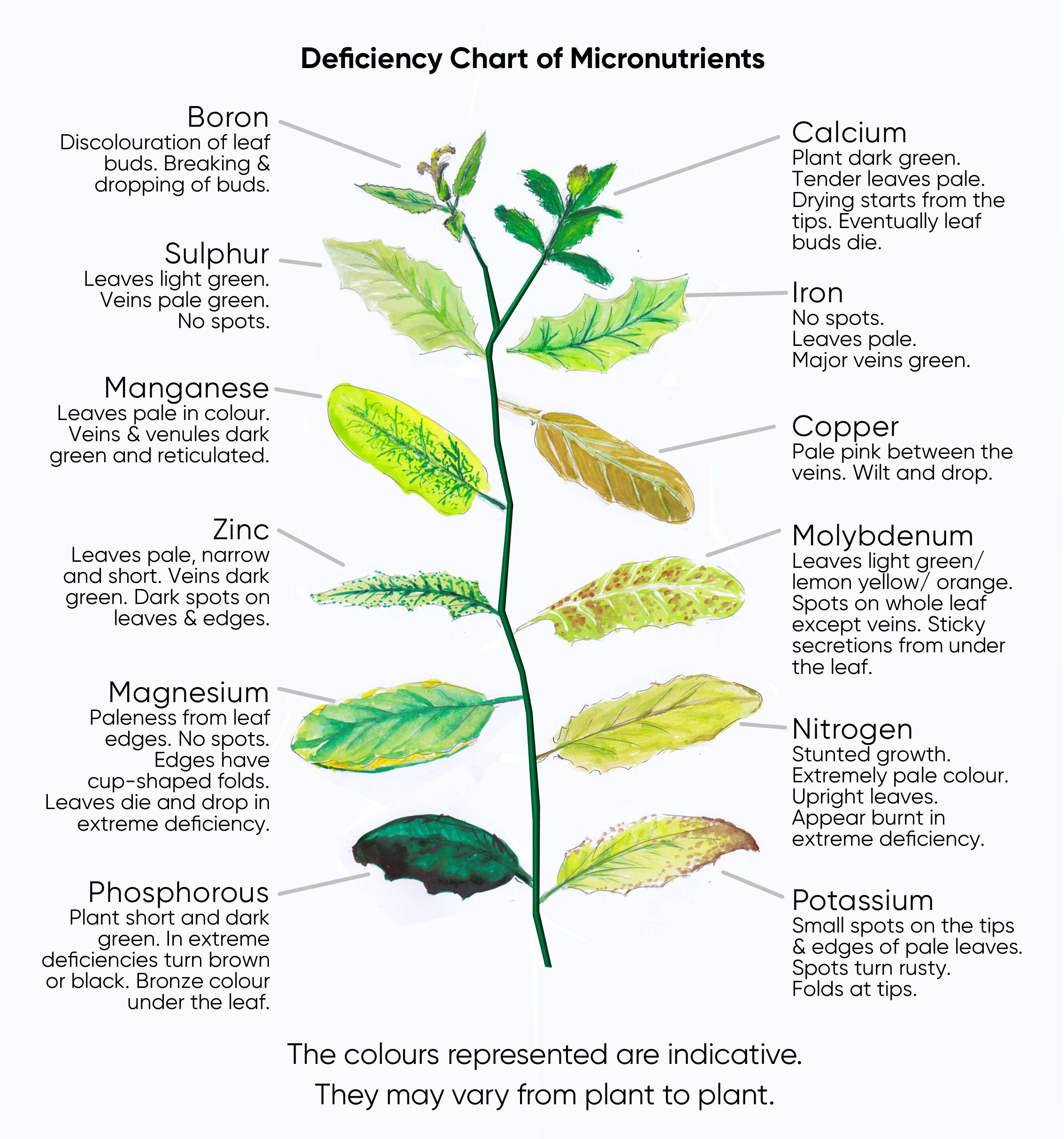
This poster is also available on the Posters tab
Sustainable Soil Health
It has been a marathon to get my head around the complexity of soils. I’ve walked away from my desk countless times, wondering how I am ever going to communicate something so intricate in simple terms. The information regarding soil fertility and nutrition is abundant and very, very scientific. pH, temperature, mineral combinations, microbes, water – so many factors affect soil health. As much of a control-freak as I am, it makes me very happy to understand that there is no better way to support soil health than by supporting nature. If we focus on feeding the soil rather than feeding the trees, the trees will find what they need. The diagram below summarises this approach.

Healthy, productive soil is not just dirt with some nutrients; it is a living ecosystem. Keeping this ecosystem alive means keeping enough organic matter in the soil to keep microbes, mites, fungi, worms and other assorted critters, who live there, happy, well-fed and able to do their jobs. For farmers, their main job is solubilising – which means freeing nutrients from their bonds (organic forms) and making them available for plants (inorganic forms).
When soils are healthy, they also suppress soil-borne pathogens such as phytophthora. I am left with no doubt that if we just keep the soil ALIVE, all the nutrients required by the trees will be made available. Obviously, some supplementation may be required as the trees deplete certain resources faster than microbes can replace them but this should be a support service to the primary business of building life in the soil. Always consider the impact of any additive on the soil life eg: copper has a major, damaging effect on soil life and care should be taken to avoid excessive use (if any) of this spray.
Nematodes and earthworms are one or two steps higher in the food chain than fungi and bacteria and are good indicators of the physical, chemical and biological health of the soil.
The fact that we want more from plants than was their intended production, we also want it quicker, for longer AND we are giving them less to get there, we create unnatural supplements to achieve those goals. These unnatural supplements cause imbalances and disturb the natural systems. It’s the inevitable messy result of well-meaning farmers chasing unnatural targets.
As this article has evolved, I have been able to equate it to my own personal nutrient choices; I know I won’t die if I only eat junk food but I also know my medical costs will probably increase and I won’t be running any marathons or producing any particularly remarkable work. My physical and mental well-being will suffer.
If I eat only whole foods, avoid anything processed, and drink enough water, I’ll be strong, healthy and well-equipped to take on any mental and physical challenges. So why are there chips in my cupboard, cheese in my fridge, wine in the rack and take-out Apps on my phone? Maybe because I don’t want to run any marathons or out-maths any geniuses. I know I may have a couple health challenges along the way but I’ve found my balance, and have aligned my input with what I expect to get out.
To take this back to mac farming … if you want the maximum quantity at the very best quality then you need world-class athletes in your orchards who never get sick and are ridiculously productive. No convenience-foods, no short-cuts, nothing processed, synthetic or even remotely toxic. Only organic, wholistic, natural goodness.
But, if you’re happy with average (which is generally what we expect from ourselves, as human beings) then convenience, cost, speed and ‘easy’ is freely available and generally comes in plastic.

Thanks to all these priceless resources without which I would still be floundering …
BIBLIOGRAPHY
https://www.geeksaresexy.net/2015/01/01/soil-made-science-video/
https://www.cropnutrition.com/nutrient-management/nitrogen
https://extension.umn.edu/nitrogen/fertilizer-urea#losing-urea-due-to-soil-temperature-and-ph-755164
https://www.sciencelearn.org.nz/resources/961-the-phosphorus-cycle
https://labmodules.soilweb.ca/nutrients-phosphorus/
http://soilweb200.landfood.ubc.ca/soil-biology/nutrient-cycles/
https://www.bbc.com/future/article/20190114-compost-or-phosphorus-fertiliser-in-africa-agriculture
https://www.farmersweekly.co.za/crops/fruit-nuts/macadamia-fertilisation-expert-guide-sa-growers/
http://era.daf.qld.gov.au/id/eprint/1964/5/mac-growing_guide_Part5.pdf
https://www.spectrumanalytic.com/support/library/ff/Ca_Basics.htm







